NEW: MVista® Coccidioides Antibody IgG IgM EIA
CLINICAL BACKGROUND
Coccidioidomycosis is endemic to the southwestern United States, northern Mexico and regions of Central and South America. Coccidioidomycosis often presents as community acquired pneumonia (CAP) and is estimated to be the cause of 15-30% of such infections in endemic areas. However, infections can range from asymptomatic to life-threatening, or acute pulmonary disease. Serology is the most common technique used for diagnosis, specifically the detection of IgG and IgM antibodies by immunodiffusion (ID) and enzyme immunoassay (EIA), followed by IgG quantification by complement fixation (CF). CF and ID are time consuming and laborious methods for laboratories, with turnaround times between 2 and 6 days. False negative results may occur in all of the antibody tests, especially in immunocompromised patients. The sensitivity of the two commercial antibody detection EIAs was estimated to be between 68% and 72% (Suneshine, R ICAAC 2014).
RESULTS
While MiraVista Diagnostics’ MVista® Coccidioides Antigen EIA has demonstrated sensitivity between 70% and 87% in immunocompromised patients and those with severe disease, improved antibody detection could increase the sensitivity for diagnosis of the cases that are falsely-negative in the antigen assay and the currently available antibody assays, especially in patients with moderate to mild disease, allowing for earlier diagnosis and treatment. In 2016, MiraVista Diagnostics developed the MVista® Coccidioides Antibody IgG IgM EIA to offer clinicians a more rapid and accurate testing method for coccidioidomycosis. The new assay has demonstrated a sensitivity of 88.3% in either IgG or IgM antibody, which is significantly higher than other commercially available tests, and specificity of 91.8%.
The EIA was positive in 31 of 41 (76%) cases in which the ID was negative. The EIA was positive in 60 of 61 cases in which the ID was positive, and was intermediate in that case. The EIA was positive in 18 of 20 (90%) cases in which the CF was negative. The EIA was positive in 39 of 41 cases in which the CF was positive, and was intermediate in the remaining 2 cases. The CF titer was less than 1:2 in one and 1:2 in the other EIA intermediate cases.
Cross-reactivity can be resolved by consideration of epidemiological factors and comparing results in the Coccidioides antibody EIA and the Histoplasma or Blastomyces antibody EIA: in the appropriate epidemiological setting, the higher antibody level is likely to indicate the correct diagnosis.
Anti-Coccidioides IgG Detection in Serum Specimens
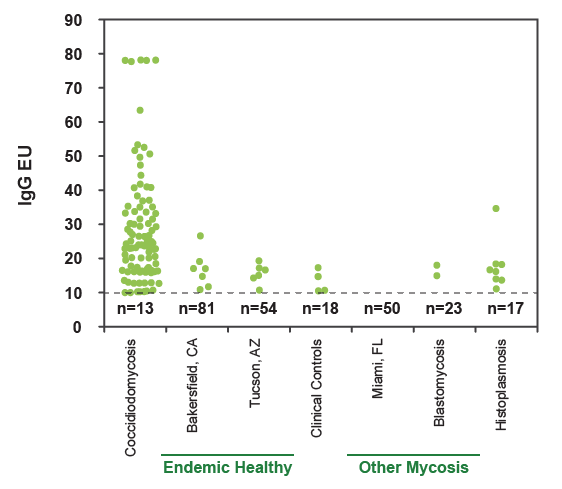
The cut-off for antibody detection (10EU) is indicated by the dashed line and the number of negative samples is indicated underneath the line. The sensitivity was 87.4% and specificity was 91.8% for detection of IgG antibodies. IgM antibodies, which are thought to be more common in acute coccidioidomycosis, were detected in 61.2% of cases.
CONCLUSIONS
The new MVista® Coccidioides Antibody IgG IgM EIA offers significant improvements in the diagnosis of coccidioidomycosis. It is a reliable method for detecting anti-Coccidioides antibodies and it is more sensitive than ID and CF antibody detection as well as other commercially-available EIAs. Additionally, MVista® Coccidioides Antibody EIA complements the MVista® Coccidioides Antigen EIA by helping clinicians accurately identify and quickly diagnose patients with coccidioidomycosis, from moderate and mild cases to severe disease.
DOWNLOAD the full details to learn more about MiraVista Diagnostics’ new MVista® Coccidioides Antibody IgG IgM EIA.