IVD Devices: Histoplasma Urine Antigen LFA
Dispositivos de DIV: EFL de antígeno para Histoplasma en orina
Dispositivos de DIV: EFL de antígeno urinário para Histoplasma
- Diego H. Cáceres, et al. Validation and Concordance Analysis of a New Lateral Flow Assay for Detection of Histoplasma Antigen in Urine. Journal of Fungi 2021.
- Wassim Abdallah, et al. Diagnosis of Histoplasmosis Using the MVista Histoplasma Galactomannan Antigen Qualitative Lateral Flow–Based Immunoassay: A Multicenter Study. Open Forum Infectious Diseases 2021.
- Martinez-Gamboa, et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: A prospective multicenter study in Mexico. PLoS Negl Trop Dis. 2021 Mar 8;15(3)
Background
Histoplasmosis is a systemic infection caused by the dimorphic fungus Histoplasma capsulatum. Rapid diagnosis is important for early initiation of treatment in progressive disseminated and acute pulmonary histoplasmosis cases. Antigen detection has been proven useful for rapid diagnosis.
Development
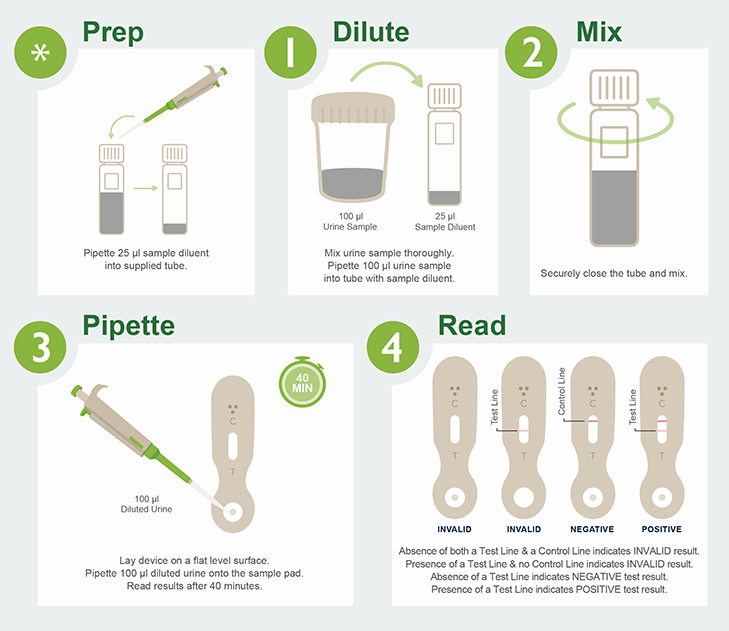
We have developed an easy-to-use immunochromatographic lateral flow assay (LFA) for the visual detection of Histoplasma galactomannan antigen in urine.
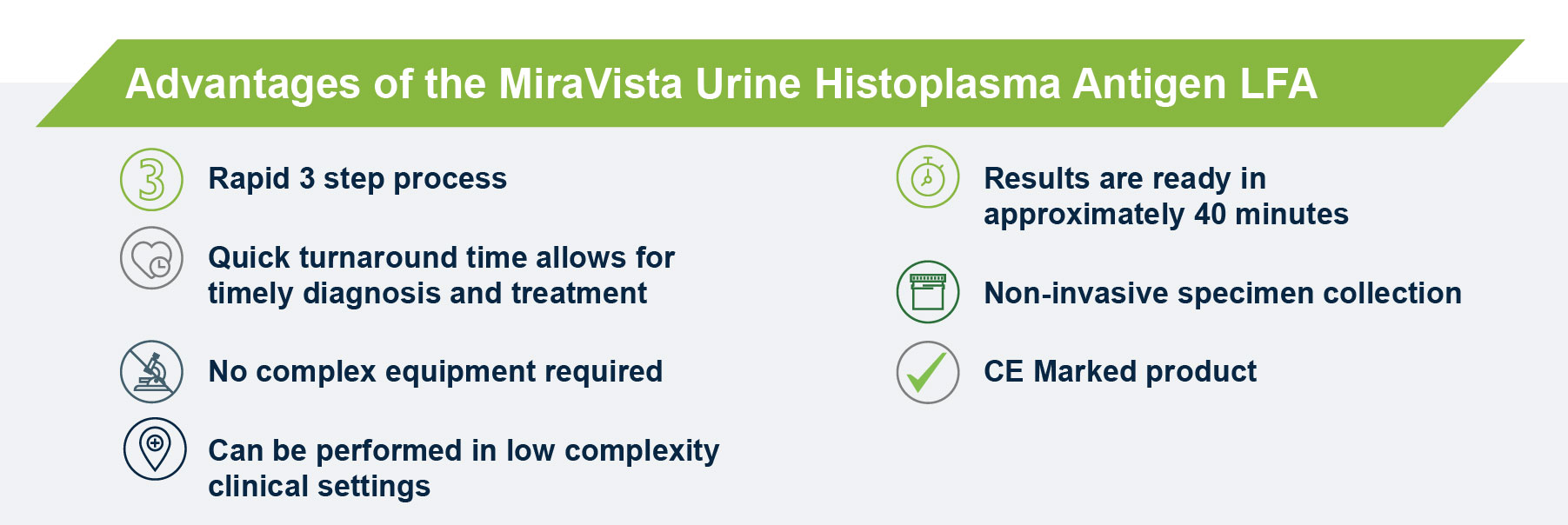
The MiraVista Histoplasma Urine Antigen LFA test can aid in the diagnosis of Histoplasma infection.
Performance
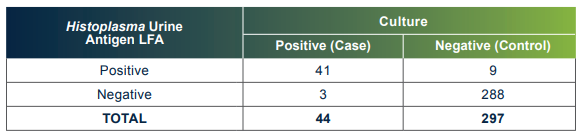
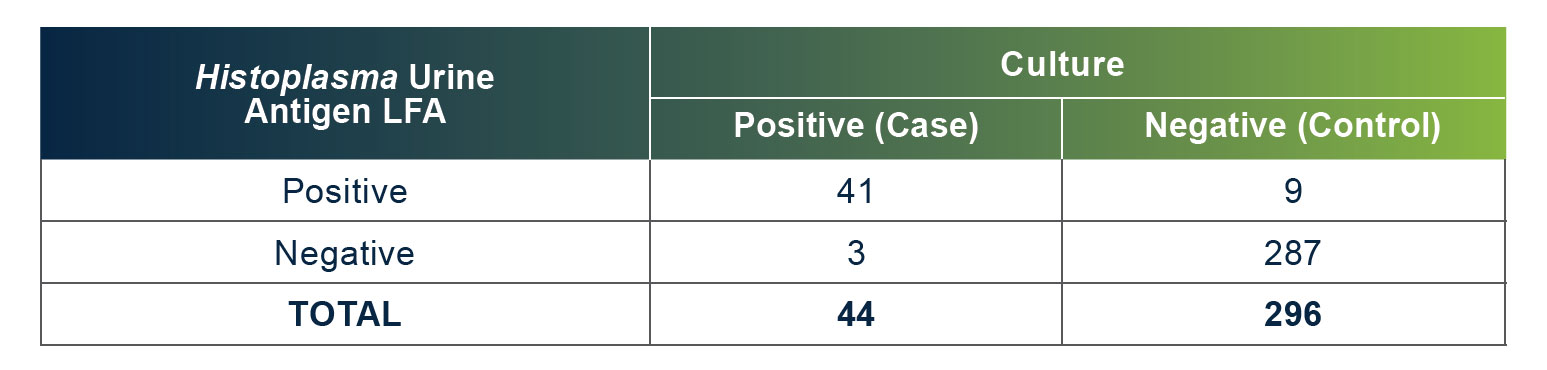
The MiraVista Histoplasma Urine Antigen LFA has a proven clinical sensitivity of 93.18% and 96.97%
Download Product Information
- Product Information Sheet
- Product Overview
- Histoplasma Urine Antigen LFA Kit IFU
- Histoplasma Urine Antigen LFA Control Kit IFU
MedicaTec SRL Av. Triunvirato 2789 (1427) Buenos Aires Argentina Ph. +54 11 4554 4600 Fax. +5411 4555 0416
Website: www.medica-tec.com Contact: Fabián Franzé Email address: marketing@medica-tec.com.ar
VM Biomedical Ltda. Avenida Presidente Kennedy, 3500 - 16° andar - sala 1609 CEP: 09572-015 - Sao Caetano do Sul – SP Brazil Ph. +55 11 5555 5408 / 55 11 9 9444 1766 WhatsApp: +55 11 9 9 8326 9681
Website: www.vmbiomed.com.br Contact: Vicente Mazzeu Email address: vendas.br@vmbiomed.com.br
- Diego H. Cáceres et al. Validation and Concordance Analysis of a New Lateral Flow Assay for Detection of Histoplasma Antigen in Urine. Journal of Fungi, 2021.
- Wassim Abdallah et al. Diagnosis of Histoplasmosis Using the MVista Histoplasma Galactomannan Antigen Qualitative Lateral Flow–Based Immunoassay: A Multicenter Study. Open Forum Infectious Diseases, 2021.
- Martinez-Gamboa et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: A prospective multicenter study in Mexico. PLoS Negl Trop Dis., 8 de marzo de 2021; 15(3).
Contexto
La histoplasmosis es una infección sistémica causada por el hongo dimórfico Histoplasma capsulatum. El diagnóstico rápido es importante para el inicio temprano del tratamiento en casos de histoplasmosis pulmonar aguda y diseminada progresiva. Se ha demostrado que la detección de antígenos es útil para diagnosticar rápidamente.
Desarrollo
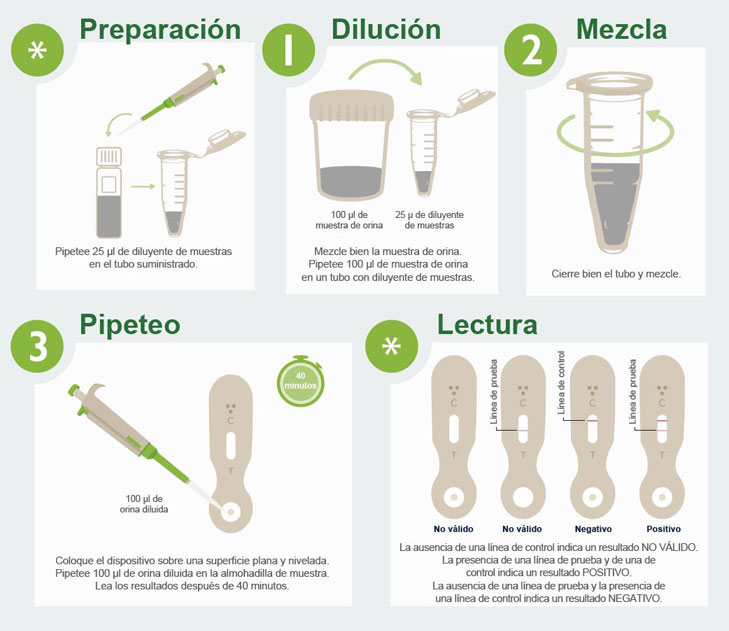
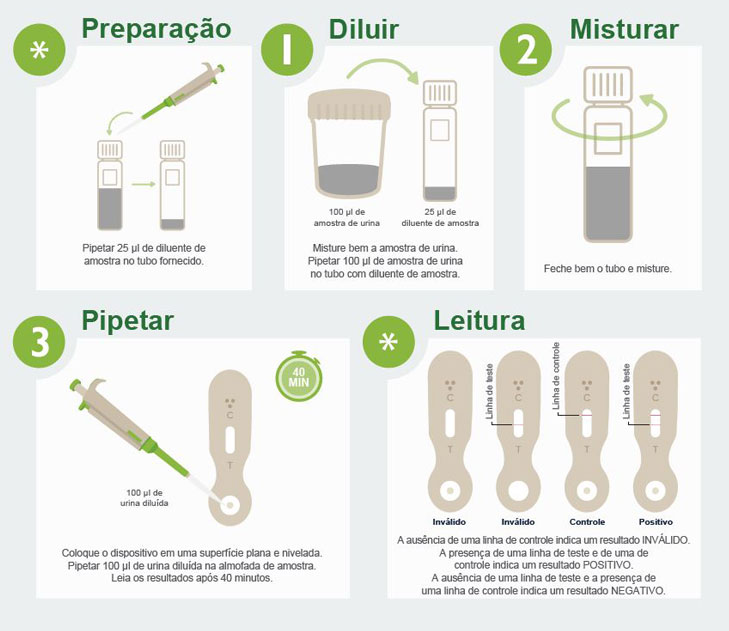
Hemos desarrollado un ensayo de flujo lateral (EFL) inmunocromatográfico fácil de usar para la detección visual del antígeno galactomanano para Histoplasma en orina.
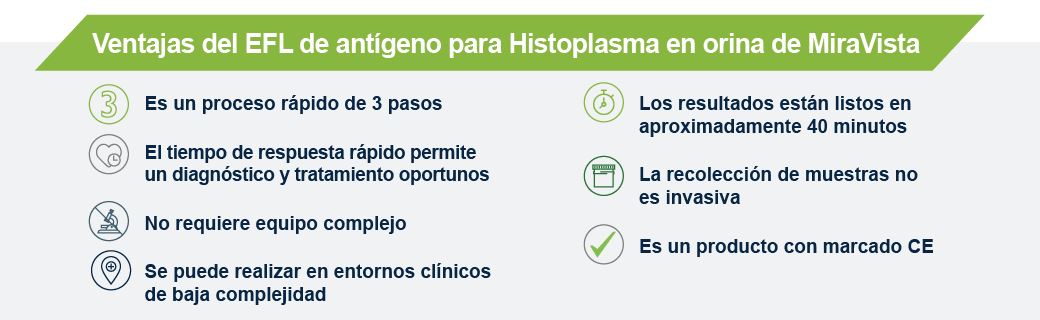
El EFL de antígeno para Histoplasma en orina de MiraVista puede ayudar en el diagnóstico de una infección por Histoplasma.
Rendimiento
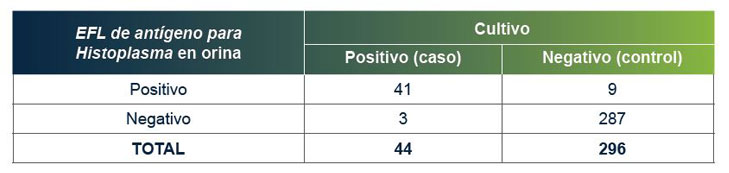
El EFL de antígeno para Histoplasma en orina de MiraVista tiene una sensibilidad clínica comprobada del 93,18 % y una especificidad del 96,97 %.
Descargar información del producto
- Hoja de información del producto
- Descripción general del producto
- Instrucciones de uso para el kit de EFL de antígeno para Histoplasma en orina
- Instrucciones de uso para el kit de control de EFL de antígeno de Histoplasma en orina
MedicaTec SRL Av. Triunvirato 2789 (1427) Buenos Aires Argentina Tel. +54 11 4554 4600 Fax. +5411 4555 0416
Sitio web: www.medica-tec.com Contacto: Fabián Franzé Dirección de correo electrónico: marketing@medica-tec.com.ar
VM Biomedical Ltda. Avenida Presidente Kennedy, 3500 - 16° andar - sala 1609 CEP: 09572-015, Sao Caetano do Sul, SP Brasil Tel. +55 11 5555 5408/55 11 9 9444 1766 WhatsApp: +55 11 9 9 8326 9681
Sitio web: www.vmbiomed.com.br Contacto: Vicente Mazzeu Dirección de correo electrónico: vendas.br@vmbiomed.com.br
- Diego H. Cáceres, et al. Validation and Concordance Analysis of a New Lateral Flow Assay for Detection of Histoplasma Antigen in Urine. Journal of Fungi 2021.
- Wassim Abdallah, et al. Diagnosis of Histoplasmosis Using the MVista Histoplasma Galactomannan Antigen Qualitative Lateral Flow–Based Immunoassay: A Multicenter Study. Open Forum Infectious Diseases 2021.
- Martinez-Gamboa, et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: A prospective multicenter study in Mexico. PLoS Negl Trop Dis. 8 de março de 2021;15(3).
Antecedentes
A histoplasmose é uma infecção sistêmica causada pelo fungo dimórfico Histoplasma capsulatum. Um diagnóstico rápido é importante para o início precoce do tratamento em casos de histoplasmose pulmonar disseminada progressiva e aguda. A detecção de antígenos tem se mostrado útil para um diagnóstico rápido.
Desenvolvimento
Desenvolvemos um ensaio de fluxo lateral (EFL) imunocromatográfico fácil de utilizar para a detecção visual do antígeno de galactomanana para Histoplasma na urina.
O teste de EFL de antígeno urinário para Histoplasma da MiraVista pode ajudar no diagnóstico da infecção por Histoplasma.
Desempenho
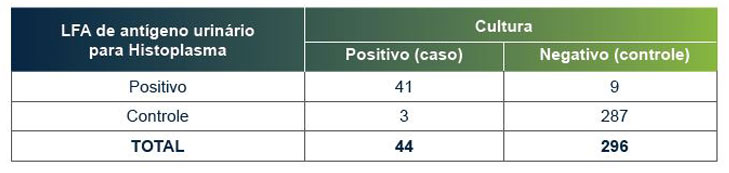
O EFL de antígeno urinário para Histoplasma da MiraVista tem uma sensibilidade clínica comprovada de 93,18% e especificidade de 96,97%.
Baixar informações do produto
- Folha de informações do produto
- Visão geral do produto
- Instruções de uso do kit de EFL de antígeno urinário para Histoplasma
- Instruções de uso do kit de controle de LFA de antígeno urinário para Histoplasma
MedicaTec SRL Av. Triunvirato 2789 (1427) Buenos Aires Argentina Fone +54 11 4554 4600 Fax. +5411 4555 0416
Site: www.medica-tec.com Contato: Fabián Franzé Endereço de e-mail: marketing@medica-tec.com.ar
VM Biomedical Ltda. Avenida Presidente Kennedy, 3500 - 16° andar - sala 1609 CEP: 09572-015 - Sao Caetano do Sul – SP Brasil Fone +55 11 5555 5408 / 55 11 9 9444 1766 WhatsApp: +55 11 9 9 8326 9681
Site: www.vmbiomed.com.br Contato: Vicente Mazzeu Endereço de e-mail: vendas.br@vmbiomed.com.br