Blastomycosis Diagnosis Case Study
Case Study:
31-year-old man from Wisconsin presented with 4 weeks of respiratory complaints and fever after fishing in the Little Wolf River. Rales were present, respiratory rate was 33 breaths/minute, oxygen saturation 88% and chest CT showed diffuse nodular infiltrates and a mass in the right lower lobe. Testing included negative immunodiffusion (ID) antibody tests for blastomycosis and histoplasmosis but positive urinary antigen tests for both.
Key Questions:
- What is differential diagnosis?
- Blastomycosis, histoplasmosis
- What further testing could be useful?
- Blastomyces and Histoplasma IgG antibody detection EIA
- What treatment is recommended?
- Itraconazole
Blastomyces dermatitidis is endemic in the Midwest, south-central and south-eastern parts of the US and Canada along bodies of water: Ohio, Mississippi, St Lawrence rivers and Great Lakes [1]. Soil containing bird guano favors growth of Blastomyces spp, and disturbances of the soil cause infection in humans and other mammals.
Culture: Culture of respiratory specimens are positive in most cases of pulmonary blastomycosis [2]. However, delays of up to 4-5 weeks can occur waiting for Blastomyces to grow [3].
Microscopy: Blastomyces’ unique features provide for an accurate rapid diagnosis in most cases using KOH preparations or calcofluor white [3].
Antigen: Results using the second generation Blastomyces galactomannan antigen detection EIA found the sensitivity to be 90% and specificity 99% in urine [4], table 2. The same galactomannan is present in Histoplasma and antigen was detected in 96% of patients with disseminated histoplasmosis. Antigen was detected in serum in 57% of patients with blastomycosis. Other specimens that can be tested include bronchoalveolar lavage fluid (BALf), CSF and other sterile body fluids. Antigen detection is the most sensitive and rapid method for diagnosing most cases of blastomycosis. Sensitivity is highest in urine. Whether testing both serum and urine increases the sensitivity is unknown. In patients who undergo bronchoscopy, bronchoalveolar lavage for bronchial aspirates should also be tested for antigen. Sensitivity was 94% [5].
Antibody: MiraVista Diagnostics recently developed an IgG anti Blastomyces detection EIA as an LDT. Validation testing found sensitivity to be 94% (N = 35) and specificity 95% (N = 100). Table 2. The question arises whether antibody detection is warranted for diagnosis of blastomycosis given the high sensitivity of antigen detection. Most publications are biased towards more severe cases accounting for the high-sensitivity of antigen detection. Others reported the sensitivity to be 76% (45/59) [7]. Blastomycosis causes more severe illness than histoplasmosis but may be asymptomatic or self-limited [8]. Antibody detection using this EIA may be the method of choice for diagnosing acute self-limited or mild disease.
Table 1. Antigen Detection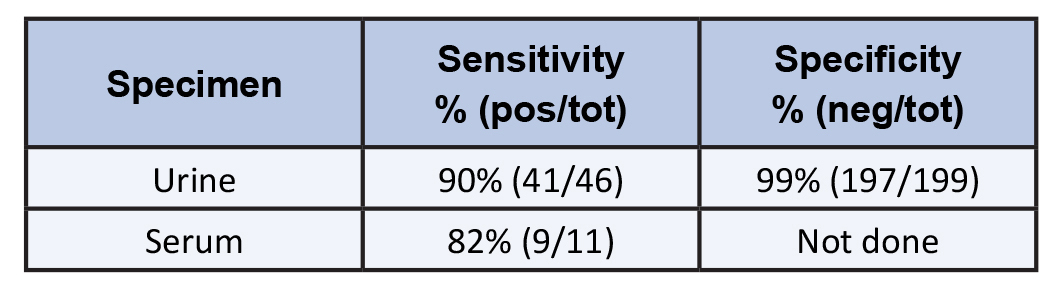
Table 2. Antibody Detection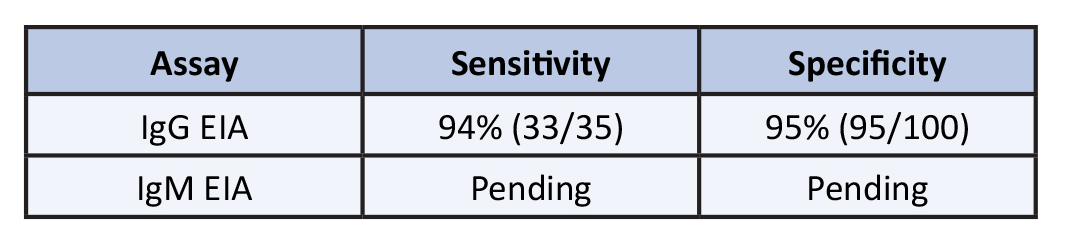
MVista®Blastomyces Antibody IgG EIA
TEST CODE: 331
CPT CODE: 86612
Clinical Significance
This assay indirectly detects anti- Blastomyces IgG by recognizing the BAD-1 repeat surface protein. During validation testing, the sensitivity was found to be 94.29% and specificity 95.00% with an assay cutoff of 14.0 EU. It offers an improvement over the current method of detecting antibodies by FID, where sensitivity has been reported as only 32%. Complement fixation is even less sensitive than FID.
The Blastomyces Antibody IgG EIA is useful for diagnosing blastomycosis in patients with negative antigen results. Secondly, when both Histoplasma and Blastomyces antigen assays are positive, comparing Histoplasma and Blastomyces antibody concentrations may assist in establishing the correct diagnosis. Additionally, following changes in antibody concentration via repeat testing may determine whether the infection has resolved or if relapse has occurred.
Methodology
Semi-Quantitative Indirect Enzyme Immunoassay (EIA)
Limitations
Specimens positive for Histoplasma capsulatum may cross-react with the Blastomyces Antibody EIA (IgG detection) and test positive (20% cross reactivity).
Specimen Collection
Serum: Collect serum specimens into a serum separator or red top tube. Allow blood to clot for 30 minutes, then centrifuge. Pipette serum separated from clot into a plastic screw cap vial.
Plasma: Plastic screw cap vial
Minimum Specimen Requirements
Serum: 0.5mL Plasma: 0.5mL
Specimen Stability
Room Temperature: 30 days Refrigerated: 30 days Frozen: 30 days
Specimen Rejection
> 30 days old
> Two unique patient identifiers are required on the specimen container.
> Any specimen type other than serum
For specimen submissions that do not meet these criteria, please call Customer Service.
Transport Temperature
Ambient/Refrigerated/Frozen
Shipping
Ship to arrive Monday–Friday using a next day delivery service. Samples may be shipped on dry ice, frozen ice packs, or ambient.
Turnaround Time
Testing is performed on Mon, Wed, Thu and Fri. Serum: Reported next day
Reference Range
Negative
Interpretative Information
Negative: <10.0 EU Intermediate: 10.0 EU – 13.9 EU Positive: 14.0 EU to ≥80.0 EU Results should be correlated with clinical presentation and history.
Additional Information
This test was developed, and its analytical performance characteristics determined by MiraVista Diagnostics. It has not been cleared or approved by the FDA; however, FDA clearance or approval is not currently required for clinical use. The results are not intended to be used as the sole means for clinical diagnosis or patient management decisions.
Complete Test Information Available
REFERENCES
-
- Smith JA, Gauthier G. New Developments in Blastomycosis. Semin Respir Crit Care Med 2015 Oct; 36(5):715-28.
- Martynowicz MA, Prakash UB. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest 2002 Mar; 121(3):768-73.
- Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc 2010 May; 7(3):173-80.
- Connolly P, Hage CA, Bariola JR, et al. Blastomyces dermatitidis Antigen Detection by Quantitative Enzyme Immunoassay. Clin Vaccine Immunol 2012 Jan; 19(1):53-6.
- Hage CA, Davis TE, Fuller D, et al. Diagnosis of Histoplasmosis by Antigen Detection in BAL Fluid. Chest 2010 Mar; 137(3):623-8.
- Richer SM, Smedema ML, Durkin MM, et al. Development of a Highly Sensitive and Specific Blastomycosis Antibody Enzyme Immunoassay Using Blastomyces dermatitidis Surface Protein BAD-1. Clin Vaccine Immunol 2014 Feb; 21(2):143-6.
- Frost HM, Novicki TJ. Blastomyces Antigen Detection for Diagnosis and Management of Blastomycosis. J Clin Microbiol 2015 Nov; 53(11):3660-2.
- Klein BS, Vergeront JM, Disalvo AF, Kaufman L, Davis JP. Two outbreaks of blastomycosis along rivers in Wisconsin. Isola-tion of Blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am Rev Respir Dis 1987 Dec; 136(6):1333-8.